Research Article - American Journal of Physiology, Biochemistry and Pharmacology (2022)
Alteration of Gastric and Salivary Secretions Due to Mosquito Coil in Male Wistar Rats
Odukanmi Olugbenga Adeola*, Salami Adeola Thabitha, Essiet Ekerette Udo and Olaleye Samuel BabafemiOdukanmi Olugbenga Adeola, Department of Physiology, University of Ibadan, Ibadan, Nigeria, Tel: +2348038224696, Email: odukanmi@yahoo.com
Received: 10-Mar-2022, Manuscript No. AJPBP-22-56852 ; Editor assigned: 12-Mar-2022, Pre QC No. AJPBP-22-56852 (PQ); Reviewed: 26-Mar-2022, QC No. AJPBP-22-56852; Revised: 31-Mar-2022, Manuscript No. AJPBP-22-56852 (R); Published: 23-Apr-2022
Abstract
Introduction: Many households in Nigeria use mosquito coils to prevent mosquito bites and this has public health implications. Despite the huge number of usage, there is no information on the effect of Mosquito Coil Smoke (MCS) on gastrointestinal secretions and this was investigated.
Methods: Thirty male Wistar rats (201.7 gb. wt ± 10.2 gb. wt) were used and the study was carried out in three phases. Rats were grouped (n=5) into 3 per phase of the experiments, group 1=control; groups 2 and 3 were exposed to mosquito coil fumes for 8-10 hours daily for 2 (2P) and 6 (6P) weeks respectively, in a well- ventilated room of 41.04 m3 incapacity. The salivary and gastric acid secretions, gastric mucus cell counts, and histoarchitecture of the stomach and salivary glands were assessed in phases 1 and 2 respectively. The salivary flow rate was determined following stimulation with pilocarpine 10 mg/kg (i.p), its electrolytes were analyzed by flame photometry method. Salivary glands were excised, weighed and histological changes evaluated. Gastric ulcer scores, acid, and mucus secretions were determined by standard procedures.
Results: Salivary flow rate (ml/min) increased significantly in the 6P group (0.06 ± 0.00) compared to the 2P (0.05 ± 0.001) and control (0.05 ± 0.001) groups. Salivary electrolytes were altered. Gastric acid concentration (× 10-6 mEq/L) increased significantly in the 2P (5.00 ± 0.30) and in the 6P (6.48 ± 0.23) compared to control (4.10 ± 0.027). Ulcer scores increased significantly in the 6P group (17.13 ± 0.43) compared to 2P (12.00 ± 0.87) and control (12.28 ± 1.81) respectively. Gastric mucus secretion (mg/g tissue) decreased significantly in 2P (10.72 ± 1.25) and 6P (12.98 mg/g ± 0.45 mg/g) groups compared to control (16.28 ± 0.26). There was a mild alteration of the histology in MCS groups compared to control.
Conclusion: Mosquito coil smokes modulate gastrointestinal secretions in the stomach and salivary glands of rats and appears toxic to stomach mucosa.
Keywords
Mosquito coil; Saliva secretion; Gastric mucus; Gastric acid; Ulcer scores; Rats
Introduction
In the tropical and subtropical regions of the world, mosquitoes are the causative agent of malaria - a public health problem in their high prevalence. The extent of the burden has necessitated the use of Mosquito Coil Smoke (MCS) as a repellent to mosquito bites across Asia, Africa, and South American [1], majorly because it is affordable and reliable [2]. This has since sprung the mosquito coil market to an estimated value in the region of one billion dollars globally, accounting for almost 12% of the universal market for pesticides in that year [3], with millions of households involved in its use. Daily exposure by an individual could be up to 60 hours per week, at night, in closed rooms with little or no ventilation. A mosquito coil consists of insecticides like pyrethrin, binders, organic fillers, dyes, and other additives that are capable of smoldering as well as burning without a flame [4].
Pyrethrins/Pyrethroids is a major insecticide component of the mosquito coil and it doubles as the most potent component [5]. Pyrethrins are non-polar [6] this makes absorption less stressful in the stomach because of the fatty nature of the gut mucosa. Absorption is faster via the oral, inhalational routes and slows through the dermal and depending on the vehicle as well. Pyrethroids are quickly metabolized in the liver. Although unlike its toxicity in the insect, pyrethrin toxicity is two thousand times lower in humans due to their higher sensitive sodium channels, lower core temperature and their small surface area are much less compared with humans [7].
Cross-sectional epidemiological studies have further implicated pyrethroids in cardiovascular damage [8], neuronal damage [9], reproductive health issues [10], etc. Pyrethroid exposure has been attributed to oxidative stress, inflammation, and DNA damage [7,11]. Animals studies have observed that exposure to mosquito coil smoke delays gastric ulcer healing from our previous study [12], other studies have implicated pyrethroid on fish [3,13], among many others.
The gastrointestinal tract secretes a diverse number of substances that are useful for various functions, such as digestion, absorption, and defence purposes. The possible effect of Mosquito Coil Smoke (MCS) on some of the secretions was investigated.
Materials and Methods
Drugs and chemicals
Mosquito coil ‘Double Rabbit’ from Gongoni Company limited were purchased for the research work. Its composition includes, d-trans-allethrin 0.2% w/w, insert ingredients 99.8% w/w.
The diameter of each mosquito coil is about 12 cm and 85 cm long and weighed about 15g. Pilocarpine was gifted from Dr. Taye Lasisi, of the Department of Physiology, University of Ibadan. Other chemicals/reagents were purchased from authenticated suppliers and were of analytical grade.
Animal grouping, husbandry, and ethical approval for the study
This study was carried out in the Animal House of Physiology Department College of Medicine, University of Ibadan, Nigeria for 2-6 weeks. Thirty Male Wistar rats (201.7 g b. wt ± 10.2 g b. wt) were randomly grouped into 3 (n=5 each, per group of a phase of the experiment), the Control, 2P, and 6P. The rats were kept in plastic cages at room temperature, with free access to drinking water and a standard laboratory rodent pellet diet. Groups 2P and 6P were exposed to MCS daily for 8-10 hours for 2 weeks and 6 weeks respectively, while the control group was left unexposed to MCS.
Each group of rats was kept in a separate room of the dimension - 3.8 m × 3.6 m × 3.0 m, with an area of 41.04 m3 with adequate air circulation. The distance between each room was about 100 m apart. Approval was sought and obtained from the Animal Care and Use Research Ethical Committee (ACUREC), University of Ibadan, (Ref. No: UI-ACUREC/18/0088). The Guidelines of the National Institute of Health on the Care and Use of Laboratory Animals [14], were observed.
Saliva collection process
After a period of exposure, the rats were fasted for 12 hours before the salivary collection procedure but had access to drinking water. The collection was conducted in the morning between the hours of 8 and 9. An individual rat was placed dorsally on an inclined plane board as previously described by Li, et al. [15], for the collection of saliva, this was done after the rats were anaesthetized with ketamine, 75mg/kg (i.p) [16]. The salivary glands were stimulated with Pilocarpine, 10 mg/kg (i.p) [17], and saliva was collected into a graduated sterile bottle for 10 min. The volume and rate of flow were determined for each collection after the expiration of the allotted time. The saliva samples were kept refrigerated at 20°C until the electrolyte composition of the saliva was assayed.
Determination of the salivary lag time
Salivary lag time was measured to determine the time at which the administered secretagogue, pilocarpine, would take effect on the individual rats in each group. It was expressed as the interval between the administration of pilocarpine and the initial salivary secretions.
Determination of salivary flow rate
The salivary flow rate was determined by measuring the volume of the saliva secreted in mL per minute.
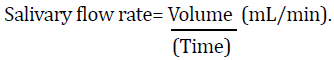
Determination of salivary electrolytes and protein
The saliva collected was analyzed for the concentrations of K+, Na+, and Cl- and HCO3-. For the determination of salivary ions, saliva was diluted at either 1/100 or 1/1000 and K+ and Na+ concentrations were determined using flame emission spectrophotometry [18]. Concentrations of Cl- and HCO3- were determined by the Schales method using mercuric nitrate [19].
Assessment of gastric mucus concentration
Adherent gastric glandular mucous secretion from each excised stomach was measured by the method of Corne et al. [21]. The excised gastric glandular portion of the stomach was transferred for 2 h to 0.1% Alcian blue dissolved in a buffer solution containing 0.1 mol/L sucrose and 0.05 mol/L sodium acetate at 5.8 pH.
The eluted dye-mucus complex that ensued was measured with a spectrophotometer and the absorption read at 605 nm.
Determination of salivary protein
Protein concentration in g/dL was measured using the Biuret reaction, as described by Dawnay et al., [20]. Saliva samples were allowed to thaw at ambient temperature. The thawed samples were centrifuged at 6000 rpm for 10 min. before use.
Determination of gastric acid secretion and collection
Gastric acid was collected by the pyloric ligation method described by Shay and his colleagues [22]. The rats were anaesthetised with a 75 mg/kg ketamine administered intraperitoneally.
The abdomen was opened through a midline laparotomy and the pylorus end of the stomach was exposed without handling the stomach. The pylorus was ligated lightly with a suture and the incision was closed. The rats were returned to their cages and re-anaesthesized 4 h after to collect the stomach contents and excised stomach tissues. The gastric contents were centrifuged at 3000 revolutions per second for 5 minutes.
The volume of gastric juice was measured in millilitres. The total acids were determined by titrating 0.5 ml and 2.5 ml of gastric juice and 0.9% N Normal Saline respectively with 0.0025 N NaOH following the addition of 2 drops of phenolphthalein solution.
The total acidity was expressed in milliequivalents of the acid and was calculated thus:
Total Acidity=Concentration (Normality of acid) × 1/1000 × 1/(Molar mass) of HCl (36.45 g/mol).
Total Acidity=Concentration (Normality of acid) × 1/1000 ×1/36.5 g/mol.
Determination of the microarchitecture of the salivary glands and stomach
The salivary glands and the stomach were excised and placed in 10% formol saline for histological analysis. The tissue was then embedded in paraffin, they were sectioned at 5 mm, and stained with Haematoxylin and Eosin (H and E) following standard procedures. Mucous cell and parietal cell counts were done with Periodic Acid Schiff (PAS) and Toluidine blue dyes respectively, and the assessment was done with the aid of pre-calibrated ToupView [R] software on the photomicrographs obtained with an Amscope camera fitted on an Accuscope microscope.
Results
Salivary lag time and flow rates in rats exposed to mosquito coil smoke
There was a significant decrease in the salivary lag time in the 6P (3.58 min ± 0.30 min) compared to the control (5.56 min ± .035 min). There was a significant increase in the salivary flow rate in the 6P (0.06 ml/min ± 0.001 ml/ min) compared to control (0.05 ml/min ± 0.001 ml/min), (Table 1).
| Groups | Salivary Lag Time(min) | Salivary Flow Rate(ml/min) |
|---|---|---|
| Control | 5.56 ± 0.35 | 0.05 ± 0.001 |
| 2P | 4.91 ± 0.27 | 0.05 ± 0.001 |
| 6P | 3.58 ± 0.30* | 0.06 ± 0.001* |
| Note: *Significant increase at P ≤ 0.05 compared with control. | ||
Saliva electrolytes composition and saliva protein concentration in rats exposed to mosquito coil smoke
There was a significant increase in the salivary sodium ion concentration (mmol/L) in the 2P (89.40 ± 0.65) and 6P (90.01 ± 0.62) compared to control (87.14 ± 1.52) (Table 2). The table also shows a significant decrease in the salivary potassium ion concentration (mmol/L) in the 2P (16.69 ± 0.93) and 6P (17.24 ± 0.16) compared to control (19.39 ± 0.45). There was a significant increase in the salivary chloride ion concentration (mmol/L) in the 2P (31.45 ± 1.19) and 6P (32.03 ± 1.54) compared to control (39.16 ± 0.90). Table 2 further shows a significant decrease in the protein concentration (g/dL) in the 2P (4.79 ± 0.03) and 6P (4.84 ± 0.11) compared to the control (5.21 ± 0.03).
| Groups | Sodium (mmol/L) |
Potassium (mmol/L) |
Chloride (mmol/L) |
Bicarbonate (mmol/L) |
Protein (g/dL) |
||
|---|---|---|---|---|---|---|---|
| Control | 87.14 ± 0.52 | 19.39 ± 0.45 | 28.16 ± 0.90 | 35.63 ± 0.40 | 5.21 ± 0.03 | ||
| 2P | 89.40 ± 0.65* | 16.69 ± 0.93* | 31.45 ± 1.19* | 36.28 ± 0.17 | 4.79 ± 0.12* | ||
| 6P | 90.01 ± 0.62* | 17.24 ± 0.16* | 32.03 ± 1.54* | 35.43 ± 0.78 | 4.84 ± 0.11* | ||
| Note: *Significant increase at P ≤ 0.05 compared with control. | |||||||
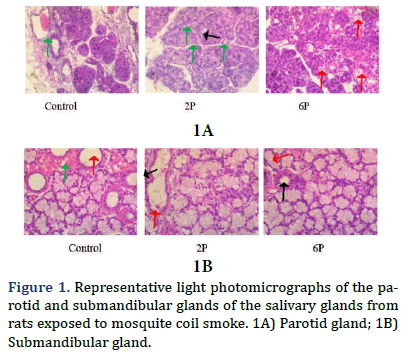
Microarchitecture of the salivary glands in rats exposed to mosquito coil smoke
Parotid gland: Controlâ?ÂÂstriated and excretory ducts contain copious amounts of secretory products (red arrows); 2P- the serous acini (black arrow) appear shrunken and slightly reduced, the striated and excretory ducts are empty. There is marked congestion of interlobular blood vessels (green arrows); 6P - there are multiple foci (red arrows) of moderate vacuolar of cells. The striated and excretory ducts are empty.
Submandibular gland: Controlâ?ÂÂthe intercalated and striated ducts (red arrows) are empty and marked congestion (green arrows) of interlobular blood vessels; 2P - there are multiple foci of vacuolar degeneration (green arrow) and necrosis (black arrow) of cells. The intercalated and excretory ducts (red arrows) contain little amounts of eosinophilic secretions; 6P - the intercalated and striated ducts (red arrow) show eosinophilic secretions.
Mosquito coil smoke on mucus secretion, parietal cell counts, acidity, and gastric ulcer scores
Table 3 describe the results of the stomach on mucus and acid secretions and mucus. Both the mucus cell and parietal cell counts increased in the MCS groups compared to the control. Apart from the significant increase observed with the gross ulcer scores, there was no significant effect that was visible on the histology slide.
| Groups | Gross Stomach | Histology | Ulcer Scores | Total Acidity (x 10-6 mEq/L) |
Mucus Secretion (mg/g tissue) | Parietal cells /UnitSq.area | Mucus cells/ Unit Sq. area |
|---|---|---|---|---|---|---|---|
| Control |  |
 |
12.28 ± 1.81 | 4.10 ± 0.27 | 16.28 ± 0.26 | 13.75 ± 0.25 | 39.25 ± 0.48 |
| 2P | 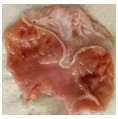 |
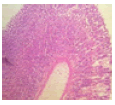 |
12.00 ± 0.87 | 5.00 ± 0.30* | 11.75 ± 1.25* | 19.50 ± 0.87* | 43.00 ± 0.82* |
| 6P | 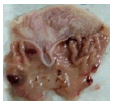 |
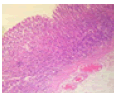 |
17.13 ± 0.43* | 6.48 ± 0.23* | 12.98 ± 0.45* | 20.25 ± 0.30* | 55.00 ± 0.16* |
| Note: *Significant change at p<0.05 compared with control. | |||||||
Discussion
The present study aimed to investigate the effects of exposure to MCS on gastrointestinal secretions (saliva and electrolytes, gastric mucus, and gastric acid secretions) of male Wistar rats. The malicious use and abuse of MCS in the tropical and subtropical regions of the world by millions of families, especially among the rural dwellers and urban poor to prevent mosquito bites cannot be overlooked. The continuous smouldering of mosquito coil constitutes a potential source of indoor air pollution [23,24].
The reduction in the salivary lag time and the increase in salivary flow rate reported in this study from the group exposed to MCS for 6 weeks post cholinergic stimulation, are of importance and should be noted for its vital essence in the possible prevention of tooth decay. The MCS potentiated the activities of the muscarinic receptors through the cholinergic agonist Pilocarpine, used in this study to stimulate salivary secretion via the parasympathetic pathways [25,26]. Reduced salivary flow rate if persistent could be a potent cause for dental caries, and other oral infections which could ultimately affect the oral health of individuals [27,28]. If nothing, good salivation plays a crucial role in digestion, especially of starchy food, and helps in the movement of food particles down into the stomach. It could not be ascertained from this study the part of the salivary fluid that increased secretion.
The salivary glands are well-known for secreting electrolytes [29]. The electrolyte composition in the saliva also varies with the type of stimulation [30]. The sodium concentration increased while the potassium level drops in the test groups. The increasing sodium ion concentration follows in a successive increase in the chloride ion within the saliva secreted. The increase in salivation has been attributed to the potentiation of the chloride channels in the apical membrane of the acinar cells during its interaction with calcium ions thereby promoting the release of sodium ions [31]. This change in composition is expected to stimulate increased salivation.
Exocytosis is a major means of the secretion of the protein from the acinar cells [30]. The autonomic stimulation aids the fusion of the vesicles for the release of proteins from the granules into saliva [32]. In the current study, the protein was reduced in the MCS groups. It is important to note that increasing stimulation does not necessarily produce a commiserate increase in protein synthesis. With the increase in the salivary flow rate, there is a relative reduction in protein synthesis, the opposite is observed with the major ionic composition.
Observation on histology from this study shows shrunken glands, especially with the parotid of the MCS groups and degeneration of the submandibular glands. This is a testifier to the exhaustion of the glands following stimulation with the cholinergic agent and potentiation of its activities by the exposure from the MCS (Figure 1).
Further on the secretions from this study, the 6P MCS groups produce more acidic gastric secretion with a decrease in mucus secretion. This was similar to our previous study where we used a chronic chemical model of gastric ulcer in rats [12], compared to the pylorus ligation model in this study. The ulcer healing was delayed in the study. This showed a similar pattern with raised ulcer scores as evidenced from both the gross scores and histology. The MCS appears toxic to the stomach also in this particular study.
The mucus layer thinned out and reduced the inherent protection of the stomach mucosa. Following the damage to the mucosa of the stomach was a reactionary increase in mucous cell count, as the mucosa begins the effort to replenish the lost mucous cells even with the persisting raised number of the parietal cells, which are the major acid secretory cells of the stomach mucosa. The parietal cells are tightly regulated through several mechanisms such as the cholinergic pathway via vagus, gastrin, histaminergic, ghrelin, somatostatin, glucagon-like peptide 1, and many others [33]. This allows the effective performance of parietal cells which include, the digestion of food, absorption of minerals, and control of harmful bacteria [33]. The proper regulation of parietal cells safeguards the appropriate secretion of HCl. When the balance is lost and there is a higher activity of its corresponding H-K ATPase pump, proton pump inhibitors are usually employed to tackle the excess.
The gastric mucous cells secrete mucous that aids protection and keep the stomach mucosa along with other protective factors in the face of the aggravating factors. In disequilibrium, it is not unusual for the cells doubling time to increase and prevent further damage to the mucosa when injured. This was observed in this study while despite the reduced mucus secretion the gastric mucous cell count increased in the MCS groups compared to control.
Conclusion
Exposure to MCS alters gastrointestinal secretions. Its effect on the salivary secretion is appearing not so damaging, the effects of its modulating activity with the gastric acid and gastric mucous secretions appear toxic to the already damaged gastric mucosa. Thereby while research continues to further elucidate the effect of MCS on the gut, peptic ulcer patients should be wary of prolonged use of Mosquito coil in malaria prevention.
Declarations
Funding/Support
There was no funding support for this study.
Conflict of interest disclosure
Authors declare no conflict of interest.
Authors’ contributions
Conception and design of study: OOA and OSB, Acquisition of data: OOA, EEU and SAT. Analysis and/or interpretation of data: OOA, EEU. Drafting the manuscript: OOA, EEU, SAT and OSB. Revising the manuscript critically for important intellectual content: OOA and EEU.
Ethical approval
Approval was sought and obtained from the Animal Care and Use Research Ethical Committee (ACUREC), University of Ibadan, (Ref. No: UI-ACUREC/18/0088).
References
- Lin TS, Shen FM. Trace metals in mosquito coil smoke. Bull Environ Contam Toxicol 2005;74:184-9.
[Crossref] [Google Scholar] [Pubmed]
- Lawrance CE, Croft AM. Do mosquito coils prevent malaria? A systematic review of trials. J Travel Med 2004;11:92-6.
[Crossref] [Google Scholar] [Pubmed]
- Ogoma SB, Moore SJ, Maia MF. A systematic review of mosquito coils and passive emanators: Defining recommendations for spatial repellency testing methodologies. Parasit Vectors 2012;5:1-10.
[Crossref] [Google Scholar] [Pubmed]
- Shu-Chen C, Ruey-Hong W, Li-Jie S, Ming-Chih C, Huei L. Exposure to mosquito coil smoke may be a risk factor for lung cancer in Taiwan. J Epidemiol 2008;18:19-25.
[Crossref] [Google Scholar] [Pubmed]
- John N, John J. Prolonged use of mosquito coil, mats, and liquidators: A review of its health implications. Int J Clin Exp Physiol 2015;2:209.
- Laskowski DA. Physical and chemical properties of pyrethroids. Rev Environ Contam Toxicol 2002;174:49-170.
[Crossref] [Google Scholar] [Pubmed]
- Chrustek A, Holynska-Iwan I, Dziembowska I, Bogusiewicz J, Wróblewski M, Cwynar A, et al. Current research on the safety of pyrethroids used as insecticides. Medicina 2018;54:61.
[Crossref] [Google Scholar] [Pubmed]
- Han J, Zhou L, Luo M, Liang Y, Zhao W, Wang P, et al. Nonoccupational exposure to pyrethroids and risk of coronary heart disease in the Chinese population. Environ Sci Technol 2017;51:664-70.
[Crossref] [Google Scholar] [Pubmed]
- Wagner-Schuman M, Richardson JR, Auinger P, Braun JM, Lanphear BP, Epstein JN, et al. Association of pyrethroid pesticide exposure with attention-deficit/hyperactivity disorder in a nationally representative sample of US children. Environ Health 2015;14:1-9.
[Crossref] [Google Scholar] [Pubmed]
- Radwan M, Jurewicz J, Wielgomas B, Sobala W, Piskunowicz M, Radwan P, et al. Semen quality and the level of reproductive hormones after environmental exposure to pyrethroids. J Occup Environ Med 2014;56:1113-9.
[Crossref] [Google Scholar] [Pubmed]
- Zepeda-Arce R, Rojas-García AE, Benitez-Trinidad A, Herrera-Moreno JF, Medina-Díaz IM, Barrón-Vivanco BS, et al. Oxidative stress and genetic damage among workers exposed primarily to organophosphate and pyrethroid pesticides. Environ Toxicol 2017;32:1754-64.
[Crossref] [Google Scholar] [Pubmed]
- Odukanmi OA, Salami AT, Olanrewaju TR, Olaleye SB. Exposure to mosquito coil smoke delays healing of acetic acid induced gastric ulcer in male wistar rats. Niger J Physiol Sci 2020;35:77-87.
[Google Scholar] [Pubmed]
- DeMicco A, Cooper KR, Richardson JR, White LA. Developmental neurotoxicity of pyrethroid insecticides in zebrafish embryos. Toxicol Sci 2010;113:177-86.
[Crossref] [Google Scholar] [Pubmed]
- Animals NR. C. for the U. of the G. for the C. and U. of L. Guide for the Care and Use of Laboratory Animals. 2011.
- Li Y, Taylor JM, Ten Haken RK, Eisbruch A. The impact of dose on parotid salivary recovery in head and neck cancer patients treated with radiation therapy. Int J Radiat Oncol Biol Phys 2007;67:660-9.
[Crossref] [Google Scholar] [Pubmed]
- Odukanmi OA, Salami AT, Isa MO, Olaleye SB. Crude ethanolic extract and fractions of Buchholzia coriacea modifies salivary secretion and electrolyte compositions in rat. Am J Physiol Biochem Pharmacol 2019;9:1-7.
- Lasisi TJ, Shittu ST, Oguntokun MM, Tiamiyu NA. Aging affects morphology but not stimulated secretion of saliva in rats. Ann Ib Postgrad Med 2014;12:109-14. [Crossref]
[Google Scholar] [Pubmed]
- Baker DE, Suhr NH. Atomic absorption and flame emission spectrometry. Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties. 1983.
- Shannon IL, Prigmore JR. Physiologic chloride levels in human whole saliva. Proc Soc Exp Biol Med 1958;97:825-8.
[Crossref] [Google Scholar] [Pubmed]
- Come SJ, Morrissey SM, Woods RJ. Proceedings: A method for the quantitative esti mation ofgastric barrier mucus. J Physiol (Lond). 1974.
- Dawnay AB, Hirst AD, Perry DE, Chambers RE. A critical assessment of current analytical methods for the routine assay of serum total protein and recommendations for their improvement. Ann Clin Biochem 1991;28:556-67.
[Crossref] [Google Scholar] [Pubmed]
- Shay H, Komarov SA, Fels SS, Meranze D, Gruenstein M, Siplet H, et al. A simple method for the uniform production of gastric ulceration in the rat. Gastroenterology 1945;5:43–61.
- Hogarh JN, Agyekum TP, Bempah CK, Owusu-Ansah ED, Avicor SW, Awandare GA, et al. Environmental health risks and benefits of the use of mosquito coils as malaria prevention and control strategy. Malar J 2018;17:1-2.
[Crossref] [Google Scholar] [Pubmed]
- Aditi Jain. Particulate matter from cigarettes, mosquito coils makes indoor air toxic. Down to Earth 2019.
- Edwards JF, Higgs S, Beaty BJ. Mosquito feeding-induced enhancement of Cache Valley Virus (Bunyaviridae) infection in mice. J Med Entomol 1998;35:261-5.
[Crossref] [Google Scholar] [Pubmed]
- Garrett JR. The proper role of nerves in salivary secretion: A review. J Dent Res 1987;66:387-97.
[Crossref] [Google Scholar] [Pubmed]
- Chávez EM, Borrell LN, Taylor GW, Ship JA. A longitudinal analysis of salivary flow in control subjects and older adults with type 2 diabetes. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2001;91:166-73.
[Crossref] [Google Scholar] [Pubmed]
- Lin CC, Sun SS, Kao A, Lee CC. Impaired salivary function in patients with noninsulin-dependent diabetes mellitus with xerostomia. J Diabetes Complications 2002;16:176-9.
[Crossref] [Google Scholar] [Pubmed]
- Busamia BE, Moles G, Mazzeo MA, Linares JA, Demarchi M, Gobbi C, et al. Assessing the determination of salivary electrolytes and anti-Ro and anti-La antibodies for the diagnosis of Sjogren’s Syndrome (SS). Med Oral Patol Oral Cir Bucal 2010;3:e437-40.
[Crossref] [Google Scholar] [Pubmed]
- Proctor GB. The physiology of salivary secretion. Periodontology 2000 2016;70:11-25.
[Crossref] [Google Scholar] [Pubmed]
- Romanenko VG, Catalán MA, Brown DA, Putzier I, Hartzell HC, Marmorstein AD, et al. Tmem16A encodes the Ca2+ activated Cl−channel in mouse submandibular salivary gland acinar cells. J Biol Chem 2010;285:12990-3001.
[Crossref] [Google Scholar] [Pubmed]
- Ambort D, Johansson ME, Gustafsson JK, Nilsson HE, Ermund A, Johansson BR, et al. Calcium and pH-dependent packing and release of the gel-forming MUC2 mucin. Proc Natl Acad Sci U S A 2012;109:5645-50.
[Crossref] [Google Scholar] [Pubmed]
- Engevik AC, Kaji I, Goldenring JR. The physiology of the gastric parietal cell. Physiol Rev 2020;100:573-602.
[Crossref] [Google Scholar] [Pubmed]